The Birth of an Insulator
by Mark Lauckner
Reprinted from "Crown Jewels of the Wire", July 2000, page 33
The way that insulators are conceived and born often result in unique
characteristics that us collectors like, (not like us collectors). From my
experience making approximately 1000 old threadless-shaped commemorative
insulator paperweights, I have witnessed all of the little problems that
produce" birthmarks" and defects in old pressed glass. As my glass
pressing techniques are as authentic as possible to the old pioneer
technologies, this has been quite an exciting learning curve. What follows is a
combination of my personal observations and some research into pioneer glass
manufacturing.
What causes leaners?
Leaners or "slumpers" are the result of one of two events. Either
the insulator was released from the mold too soon and shifted in shape a bit
before rigidizing, or the temperature during the annealing process was too high
and the glass sunk a little under its own weight.

A leaning two-tone
CD 121 Pleated Skirt
What causes underpours?
What we call "underpours" are hardly ever caused by an insufficient
volume of glass in the mold. They are usually caused because the glass cools
before it can be squeezed into the small areas of the mold. This is why underpours occur on the base and inner skirt region. This is the area
where there is the most amount of mold around the smallest amount of hot glass.
In old insulators, the plunger depth or skirt length compensated for the different volumes of glass portioned for each pressing. This results in a deeper
pinhole or shorter insulator if there is a smaller portion of glass in the mold,
so a true "underpour" is a very rare occurrence.

Underpoured base on a
CD 161 California.
What causes snow in insulator glass?
Snow is small particles of eroding furnace brick which has broken up and
flowed out in the glass tank. The glass tank bricks were usually a high alumina
firebrick, so the main component in the snow would be clay particles. (Modern
glass tanks are made from fused zirconia brick.) If the particles are larger
than a sesame seed, they can cause fractures because the glass contracts around them during cooling. This usually only
affects the area close to a surface. "Pot stones" are simply larger
pieces of eroding furnace brick becoming suspended in the glass. Pot stones the
size of a dried pea are usually significant enough to always cause a fracture
during cooling.

This CD 145 H.G.CO. beehive
looks like a snowy blizzard.

A huge pot stone in the dome
of a CD 152 Brookfield.
How do objects get inside insulator glass?
Metal objects like nails, coins, wire, and bottle caps have been found in
insulators. Metals with a higher melting temperature than the glass will remain
intact for a while, but would eventually be dissolved by the hot caustic nature
of the glass fluxes. These objects are everyday types of junk, and were probably
introduced into the glass tank through shoveling cullet (broken bottles, one
with cap still on neck!), floor sweepings, etc..

Skirt length bent nail in a
CD 102 N.E.G.M. Co.

A 1920 Lincoln penny in the
dome of a Hemingray-42.
What causes creases and lines on insulators?
These are sometimes referred to as "straw lines" or "cold
mold" and are lines in the glass surface where cooling has occurred during
the pouring and the pouring stream did not completely melt back together during
the squeezing. The liquid glass folds over itself as it fills the mold and the
creases are hardened in position when they touch the-sides of the mold. The
glass does melt back together on the inside, but because the outer glass is
contacting the (cooler) mold parts, the glass can't retain the heat needed to
completely fuse back together again on the surface.

Straw lines on this cornflower
blue CD 106 Duquesne show up
on the skirt below
the embossing.
"Cold mold" is something entirely different. If the mold is not at
least 1000° F., then the glass stream entering the mold will cool instantly as
it flows over itself. This causes a concentric rippling effect, much like
throwing a stone into a pond. They are occasionally seen on the domes of
insulators, but are not caused the same way as creases and folds in the glass.
The parallel thin lines which are often seen over the dome and down one side
of an insulator are from a long thin "stringer" which enters the mold
before the rest of the pour. This only occurs when multiple insulator molds were
being filled from the same ladle, because the glass never actually breaks clean
at the end of a previous pouring. It just hardens with a little thread on the
end, which becomes the first part to enter the next mold as the pouring begins again. This is
evident on lots of H.G. Co. signals and on lots of Brookfield products, too. The
thin parallel lines can be followed until they branch away from each other. This
is the point where the hardened stringer on the bottom of the pour is attached
to the hotter contents of the ladle.

Concentric rippling of the dome
glass on the CD 145 H. G. Co.
Petticoat
formed when the
molten glass was poured into
a mold that is cold.

Parallel lines of a stringer on front
of an H.G.Co. signal's dome.
Later, with automatic portioning machines,
the glass stream was cut or sheared off for each mold filling. This often left a
shear mark about 3/4" long somewhere on the bottom of the mold (on the
dome). Lots of the later era glass insulators have this marking on one side of the dome, like Dominions, Whitall Tatums, Armstrong, etc.
What are those little drip points on some inner skirts?
These are air vents. Little holes are drilled into mold parts which have the
potential to trap air during the pressing. The inner skirt is often the only
place on the pressing where there is a surface with no moving mold part. The
small spaces between moving mold parts allow trapped air to escape during the
squeezing of the glass in the mold. Several styles of Brookfield insulators were
pressed in this type of mold, and no mold line can be found on the inner edge of
these inner skirts.

The inner skirt of a Brookfield signal
showing the small air vent drip point.
Most of these occur in each of the four
quadrants of the inner skirt's
circumference.
What causes amber streaks?
Amber streaks are caused by iron oxide (rust) or ferrous containing metals in
the glass. Just one little fleck of rust or scrap iron causes a nice amber
streak when poured. If rusty steel or iron ladles were used to pour insulators,
rust flecks would be left in the glass tank after each time the ladle was dipped
in. These flecks would melt and make little regions of dark green glass waiting
for the ladle to come back and scoop them up for the next pour. Iron oxide is
one of the oxides used for coloring glass green. When we refer to amber
streaking in glass, it's usually green that we see.
What causes milk streaks?
"Milk" or non-transparent streaks are the result of accidental
contamination by a chemical oxide which acts as an opacifier in the glass, or
the deliberate addition of these chemicals in the previous furnace batch to
produce opalescent or opaque glass. (See two-tones.) Small milk streaks in an
insulator would certainly suggest undesired contamination in the glass tank,
while the fully swirled milky insulators (like the Hemingray-9 and 12's) would
suggest that transparent glass was loaded on top of a previous run of opaque or "milk glass", and that some mixing
action in the glass tank had partially blended the two together.

A radical amber streak in a
CD 162.5 PRR (dome embossed)
made by Brookfield.
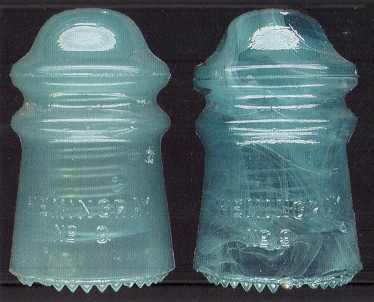
Jade milk and milk-streaked Hemingray - 9's (CD 106).
The chemicals
used as opacifiers in glass were usually calcium fluoride (fluorspar), fluorine,
calcium phosphate, or sodium phosphate. Nowadays, to obtain the right balance of
phosphorous oxides in the glass, tri-sodium phosphate (TSP) is the main
opacifier used. The fluorides and fluorines are used to produce the opalescent
glasses which have an orange-ish tint when held up to a light source (will pass
light like the opalescent Hemingray and Fry insulators). A little TSP in aqua
glass would result in the "jade milk" Hemingray products.
What causes two-tone colors in insulators?
Two-tone insulators are the result of one color of glass being loaded into an
almost empty furnace on top of another color. When the area lower in the tank is
scooped out to pour insulators, the region where the 2 colors meet can be the
gathering area for the ladle. The pour then enables both colors to enter the
mold at the same time.

A Hemingray CD 162 signal
with a two-tone mixture of
aqua and clear glass.
What causes vapor bubbles in glass?
When little flecks of metals (with lower melting temperatures than glass) get
into hot glass, they melt and emit a vapor, causing a little bubble around the
fleck. This burns off within a few minutes, and the bubbles rises to the surface
of the glass pot and pop. Various metals melt within the temperature range of
liquid glass (2400° F.).
When flecks of metals get imbedded in hot glass, the vapor pressure of the
evaporating metal causes a bubble. When the glass cools, the metal vapor
condensates on the inside of the bubble cavity. The metals condensate back to
their original colors, for example, aluminum looks like shiny silver and copper
looks dull reddish-brown.
What causes black bubbles in glass?
Bubbles with black sides are carbon or graphite. They would be from sawdust
or small wood chips getting trapped in the hot glass. As they burned, a gas was
released causing the bubble. When the glass cooled, the vapor condensated and
deposited graphite (carbon) on the inside of the bubble.

Huge black bubble in the
skirt of a CD 126.1 Brookfield.
What causes bubbles in glass?
Tiny "seed" bubbles are the result of incomplete glass ingredient
melting. The alkali which dissolves the silica in the melting action
creates small gas bubbles. These bubbles take a long time to rise to the surface and pop, and usually become suspended in the
molten glass. They can be squeezed out by reducing the temperature in the
furnace and then raising it again. This is referred to as "pinching"
the glass, but wasn't done very often with old insulator glass because optical
clarity wasn't required.

Seed bubbles throughout a H.G.Co.
Petticoat beehive (CD 145).
For any bubble bigger than a seed bubble to be trapped
in glass, the air must have been trapped very close to the time the glass was
being poured and cooled. Most bubbles are trapped during pouring and are pressed
into place as the glass cools in the mold. In the furnace, large bubbles would
rise and pop on the surface, so no large bubbles would exist in the glass
supply.

This bubble is about the size
of half of the skirt. Not sure
how the piece
screwed on a pin,
for the inside of the airspace is broken.
What makes color in glass insulators?
Metals, mostly metal oxides, produce color in glass.
Unintentional coloring:
This is the result of melting raw minerals contaminated with trace metal ores.
It can also be the result of contamination from the melting equipment. A good
example of this is the pioneer blackglass. This glass is actually very dark
green and is the result of heavy iron contamination. Melting glass in an iron
furnace pot produces this oversaturated "blackglass". The more common
accidental coloring is the result of excavating silica sand deposits
contaminated with iron and copper oxide impurities. Mined silica sand for glass
making needs to be extensively washed and cleaned in order to produce good
quality glass. This simply wasn't required for insulator production. Varying
degrees of iron and copper oxides produce the vast array of aquas and greens we
see in insulators.
Intentional coloring: Perhaps the most common intentional coloring of pioneer
insulators would be cobalt blue and amber. These insulators are usually from
glass companies using bottle glass to press insulators. Cobalt oxide is added in
very small amounts to glass ingredients to produce the cobalt blue color. It
produces cornflower blue in very, very tiny amounts. Amber is produced with
sulphur and iron oxide. Amber and cobalt bottles were intentionally made for
medicines and liquids which required some UV protection.

Some blue and amber insulators were marketed in the early 1900's as line
markers and would have been intentionally colored. These H.G.Co. and Hemingray
insulators wound up being various shades of yellow, amber, red amber, brown
amber and also electric blue, peacock blue, and various cobalt blues. Yellow can
be produced by adding sulphur to the glass batch. Very small amounts still
produce the yellow color. It's an inexpensive additive and is widely used.
Larger amounts mixed with small amounts of iron oxide produce the golden, red;
and deep root beer ambers, while the electric blue is achieved with copper
carbonate. Turquoise blue is the result of mixing small amounts of cobalt oxide
with the copper carbonate. Hemingray blue requires slightly more cobalt in the
copper carbonate. Iron oxide (better known as rust to all of us who own older
vehicles) by itself produces emerald-olive green. Copper oxide produces, a pea
soup green which is slightly opaque (jade milk glass).
(Photographs provided by Dwayne Anthony. Gary Kline and John McDougald.)
| 